Medical / Healthcare
Stable Runtime
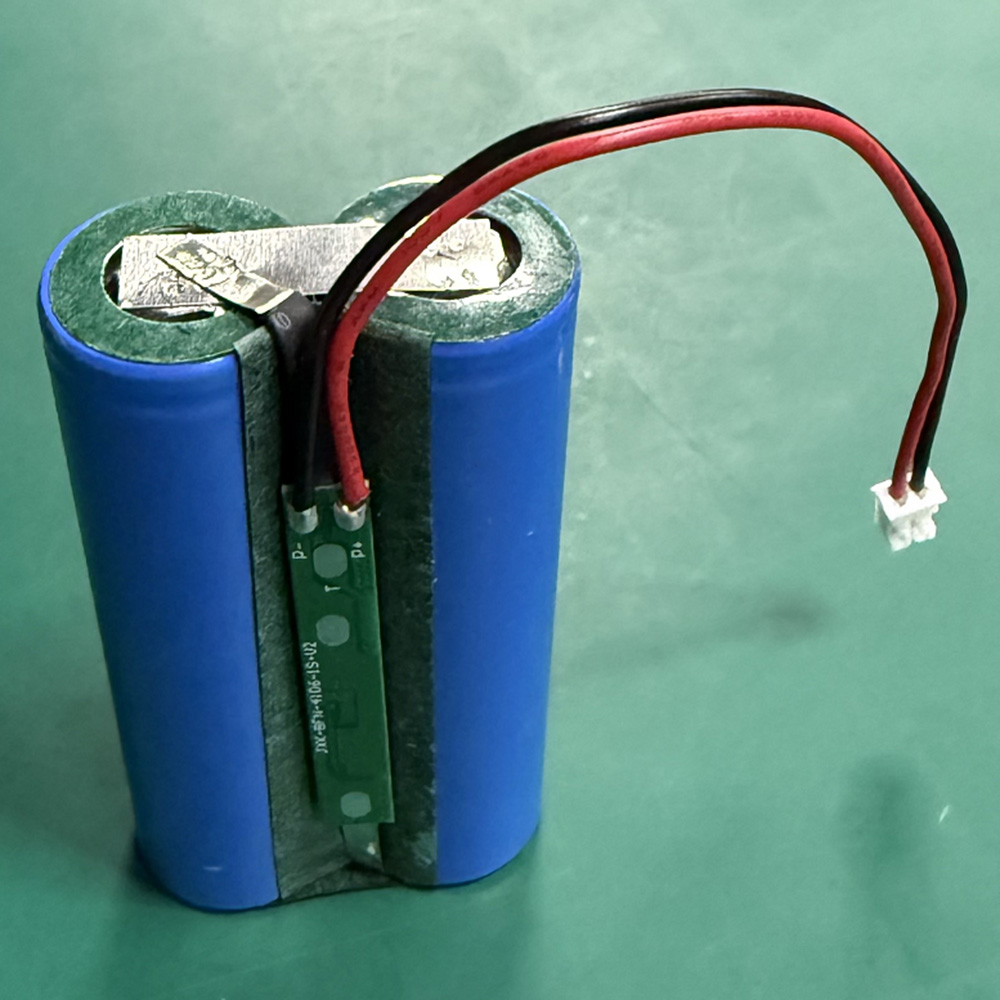
3.7V 4400mAh INR18650 Battery Pack for Medical Monitoring Instruments
A medical device customer needed a compact battery pack to power a monitoring instrument with
stable voltage, predictable runtime, and safe charging/discharging limits.
PKNERGY engineered an INR18650-based 3.7V 4400mAh pack with controlled internal resistance and
production-ready assembly to support reliable operation in clinical environments.
plus consistent pack assembly and clear operating thresholds to reduce field issues.
Medical-grade consistency
Clear cut-off limits
Compact size
Introducing the Customer (Anonymous) & Their Journey
The end-customer is a medical device manufacturer developing compact monitoring instruments for clinics and care facilities.
Due to confidentiality, we do not disclose the company name. For this case study, the project representative is described as a realistic anonymous profile.
Charge: 10°C to 35°C (recommended)
while keeping the pack compact and easy to integrate into production.
Challenges (What the customer needed to solve)
stable voltage behavior, clean assembly, and predictable cut-off limits help reduce field risk.
Solution (How PKNERGY engineered it)
PKNERGY delivered a compact INR18650 battery pack built for monitoring instruments.
We focused on stable output, safe thresholds, and production consistency—so the customer could integrate smoothly and
maintain predictable runtime across batches.
Technical Specifications
Discharge Temperature: -20–0°C (0.2C) · 0–10°C (0.5C) · 10–35°C (3A) · 35–60°C (0.5C)
Results & Customer Feedback
- Stable device operation: predictable voltage and runtime for monitoring workflows.
- Cleaner integration: compact size and connector layout simplified enclosure installation.
- Reduced field risk: defined cut-off limits improved control behavior and safety margins.
- Production consistency: repeatable assembly and QC checkpoints supported batch supply.
PKNERGY provided a solution that integrated smoothly, delivered consistent performance, and gave us confidence in batch repeatability.”
Photo Gallery (Optional)
Replace each image URL with your WordPress Media Library link. Duplicate or remove cards freely.
FAQ — Common Questions
Our engineers will propose a production-ready battery solution.
Post time: Jan-06-2026